A prior posting described the “track-and-trace” program that California plans to implement to regulate and tax the cannabis market. Each step in the supply chain needs to be documented, and the parties to any transaction are required to be licensed by the Bureau of Cannabis Control (BCC). Recall that sampling must be done by employees of the lab, and that the lab must do all the work, including all the assays.
Running all the required assays presents a large problem for a start-up lab. Even well-designed and well-documented analytical protocols, such as provided by AOAC, U.S. FDA, ASTM International, and the U.S. Pharmacopeia (USP), must be practiced and validated in the lab with the lab staff. Plus, standard operating procedures (SOPs) need to be created and adopted, and employees must be trained.
My impression is that California intends to set the standard for effective regulation of the cannabis market segment. The state is the largest domestic producer of illicit cannabis and medicinal cannabis. This experience base should be effective in assuring the public that the products and transactions involve products that meet their expectation of a known and reproducible risk profile. Ideally, it should replace the illicit market with a licit one.
Now, let’s see what items pertain to the cannabis laboratory.
SOPs for the laboratory
Section 5292 of the California emergency procedure lists requirements for Standard Operating Procedures for Laboratory Processes. The laboratory is responsible for developing, implementing, and maintaining written SOPs for the following functions:
- Calibration and maintenance of equipment and instruments
- Chain-of-custody protocols
- Data review and internal review process
- Analytical methods
- Employee training
- Premises and sample security
- Quality assurance and quality control procedures
- Recordkeeping and retention
- Sample preparation
- Sample storage
- Audit protocols and corrective actions
- The laboratory director shall review, sign, and date each SOP, including revisions
- SOPS shall be available to laboratory and field staff and the BCC.
Analytical methods
In contrast to “track-and-trace,” the regulations issued on November 17, 2017 do not specify the analytical methods and technology. Apparently, the labs are faced with using methods from ASTM, USP, and other sources. SOPs for analytical method 5295 require the laboratory to make measurements using equipment and methods that have been tested to ensure that they are fit-for-purpose of the test. The BCC recognizes several sources of methods, including: 1) the U.S. FDA’s Bacterial Analytical Manual, 2016; 2) AOAC International’s Official Methods of Analysis for Contaminant Testing of AOAC International, 20th Ed., 2016; 3) Methods of analysis for contaminant testing published in the 2016 United States Pharmacopeia and the National Formulary (USP-NF); and 4) if a laboratory wants to use an alternative scientifically valid testing methodology, it shall validate the method and submit it to the BCC.
Validation requires:
- Identification of the assay
- List of analytes
- Applicable matrices
- Method sensitivity (operating range)
- Potential interferences
- Analytical instruments
- Operating supplies, including reagents, standards, and SPE cartridges
- Sample preservation, storage, and hold time
- Protocol for QC samples, including acceptance criteria
- Protocol for calibration standards, including acceptance criteria
- Protocol for running the assay, including samples, QC standards, and calibrants
- Data quality assessment and acceptance criteria
- Calculation of results
- Preparation of reagents, solutions, and reference materials.
Required assays for cannabis products (section 5304)
The minimum testing panel shall include cannabinoids (identification and amount), residual solvents and processing chemicals, pesticides, microbiological impurities, mycotoxins, water activity and moisture content, filth and foreign material, and heavy metals. The cannabinoid assay must include THC, THCA, CBD, CBDA, CBG, and CBN to three significant figures. A 15% tolerance band is accepted between the label and the measured potency. Terpenes need only be tested for if the cultivator, manufacturer, or distributor wants to claim on the labeling that the medical cannabis goods contain terpenes for any reason.
Specific assays
Residual solvents (section 5310)
The action level for residual solvents in manufactured cannabis batches is given below. Chemical names and CAS numbers are for identification and cross-reference. The action level for inhalation products is often much lower than infused products. No assays are required for dried cannabis flower, kief, and hashish goods.
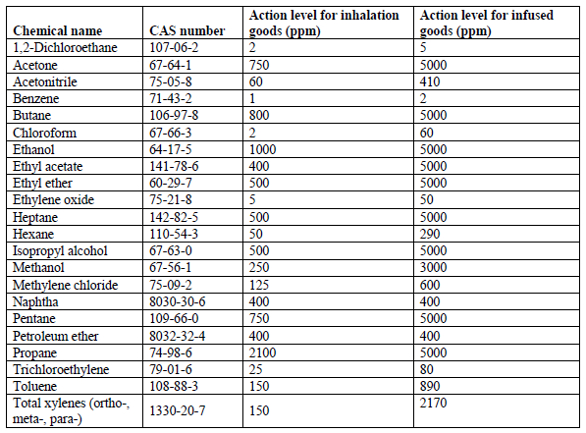
Residual pesticides (section 5313)
The BCC regulations stipulate that the laboratory shall report assay result to three significant figures in the Certificate of Analysis. If a sample contains pesticide above the listed level, the sample and batch fail, and cannot be released for retail sale.
The BCC document does not list analytical methods for pesticides, including residues. However, ASTM does provide methods for pesticide residues using GC/MS. The FDA Pesticide Analytical Manual published in 1999 is old but still useful. Restek (Bellefonte, PA) also provides pesticide standards and supporting application notes.

Biological contamination
Section 5316 deals with specific microbiological toxins Salmonella and Aspergillus. The FDA’s Bacterial Analytical Manual, 2016, is a place to start. Detection limits are generally nondetect in one gram of sample. Samples failing microbiological specifications shall be noted on the Certificate of Analysis and shall not be released for retail sale.
Mycotoxins
Protocols for assay of mycotoxins are included in the ASTM compendium described above, as well as the FDA’s manual, above. The laboratory is required to test for mycotoxins with technology with an action level of less than 20 µg/kg for aflatoxin B1, B2, G1, and G2 and ochratoxin A.
Water and dryness
Water activity is governed by section 5322. This section is confusing and should be consulted when designing the fit-for-purpose test protocol. When a sample for water content fails a test, the batch can be reworked to improve acceptability of the results. Typical methods involve drying to constant weight. I’m impressed with the Karl Fischer thermal desorption system from Metrohm USA (Riverview, FL), which uses a heating block to warm the sample as dry nitrogen is swept through the sample chamber. The water is carried over to the titration cell, which is filled with Karl Fischer reagent for titration. This simple device avoids problems of the titrants reacting with the plant material.
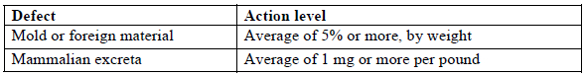
Filth and foreign material (section 5325)
The requirement is listed below, but the lab needs to develop, document, and validate the specific protocol.
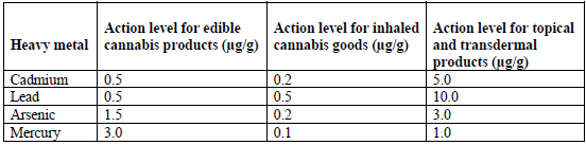
Heavy metals
Analysis of heavy metals is one of the required panels of tests for each batch of cannabis and cannabis products. The FDA recently published an update to its Elemental Analysis Manual for Food and Related Products that includes discussion instruments for inductively coupled plasma and atomic absorption spectrometry.
Terpenes are an optional assay that can be ordered by the owners of the batch (section 5331).
Other cannabinoids
The laboratory will report any sample containing synthetic cannabinoids as “failed.” For samples that fail any laboratory test, a copy of the Certificate of Analysis will be sent to the track-and-trace system of the BCC within two business days. Samples that pass testing will be entered into the “pass” track-and-trace system. The batch may be released for retail sale.
Laboratory waste and failed batches
A laboratory shall discard hazardous waste, including hazardous waste containing cannabis, in accordance with federal and state hazardous waste laws (section 5343). However, do such regulations exist for cannabis samples? At the federal level, one cannot expect cooperation. The Bokashi process has been engineered to process marijuana waste on the scale of 0.5 to 12,000 tons per month.
Comments
The test panel required by the BCC is extensive. Labs need to select from several options and reduce published methods to practice, with trained staff.
I compared the BCC regulations to Oregon’s published rules. California requires more. For example, lab waste needs to be compliant with all regulatory bodies. In California, batch size is limited to 10 pounds rather than 15 in Oregon. Lab waste, including failed batches, needs to be covered by a disposal plan. Batches that test positive for synthetic cannabinoids in California are to be destroyed. To preserve impartiality, lab owners should not have significant financial interests in other enterprises involved in the growth/distribution or sales of cannabis products.
This raises the question of cost to comply with the California program. The laboratory work will have a significant cost per batch. As mentioned above, California’s emergency regulations limit batch size to 10 pounds. This has a retail value of about $50,000. In Oregon, I was quoted a price for a THC panel consisting of THC assay, dry weight, and pesticide residue of $400, including local sampling. However, this did not include duplicate field samples.
So, let’s pick a number of $1000 for the lab work in California. This is only 2% of the retail price, assuming a retail value of $50,000/batch. The retail price will include an excise tax of 15% (~$7500). Add that to the lab cost for a total overhead of $8500. Then there is the cost of the dispensary, including rental of a store front, staff (security, advertising, promotion), plus compliance staffing the track-and-trace license system. I could see that this might cost $10,000 per batch. These overhead items associated with licit growing and distribution are largely absent with the street dealer. It seems that street vendors may be able to undercut the retail price and still have a good profit margin.
It will be interesting to compare the value that customers will place on the licit distribution (BCC-controlled) channel. If consumers do not appreciate the value, then the illicit street market will continue to thrive by being price-competitive. We are living in interesting times.
Robert L. Stevenson, Ph.D., is Editor Emeritus, American Laboratory/Labcompare; e-mail: [email protected]