During any extraction process, interferences can also be extracted along with desired analytes. These unwanted coextractables can interfere with analyte detection or decrease instrument performance. Traditionally, chromatographic techniques such as gel permeation chromatography (GPC) or a glass column packed with specific adsorbents are used to purify sample extracts prior to separation and analysis.

Figure 1 - Extraction cell with flow-through design.
The Accelerated Solvent Extraction (ASE®) system (Dionex Corp., Sunnyvale, CA) uses elevated temperatures (up to 200 °C) to increase the kinetics of the extraction process while applying high pressures (1500 psi) to maintain the organic solvents in their liquid state. The sample is enclosed in a stainless-steel extraction vessel that is automatically filled with solvent and is then heated to the preset temperature under high pressures for a static period of time (5–10 min). Once the extraction is complete, the cell is flushed with fresh solvent and then purged with compressed nitrogen to dry the sample. The extraction solvent and analytes are delivered into a sealed collection vessel. The flow-through design of the ASE system facilitates selective removal of interferences during sample extraction, thus combining extraction and purification into a single step, as shown in Figure 1.


Figure 2 - Extraction of PBDEs from salmon with (extract on left) and without (extract on right) in-cell cleanup.
For example, adding alumina to the extraction cell before adding the sample or sample mixture has been shown to prevent the extraction of unwanted lipids. There are many different adsorbents that can be used in the extraction cell to remove a wide variety of interferences from the final extract. Table 1 lists common adsorbents that are used for selective extraction of compounds during an ASE extraction.
Figure 2 shows two extracts of 10-g samples of salmon tissue. The yellow extract was obtained using standard ASE conditions (hexane, 100 °C, 10 min) and without any sorbent in the extraction cell. The clear extract was obtained from a second sample extracted under the same conditions but with alumina and sulfuric acid-impregnated silica gel in the extraction cell. The GC-MS analysis of the clear sample showed that no lipids are present in the extract. The following examples illustrate other cases using the in-cell cleanup capability of ASE.
Selective extraction of PCBs from various fat-containing samples
Selective extraction of PCBs from fish meal samples using acid-impregnated silica gel5,6
Aliquots of fish meal samples (2 g) were ground together with sodium sulfate (2 g) and Ottawa sand (2 g) using a mortar and pestle. The sample mixtures were spiked with cod liver oil CRM 349. The spiked sample mixtures were added to an ASE extraction cell that contained layers of silica gel and acid-impregnated silica gel (see Figure 3). The samples were extracted automatically using the ASE system using 100% n-hexane at 100 °C with two cycles of 5 min each. The extracts were clear and required no postextraction cleanup steps. The clean extracts were concentrated to 1 mL and diluted to 2 mL with n-hexane and then analyzed using the GC-MS. The PCB recovery data can be found in references 5 and 6.
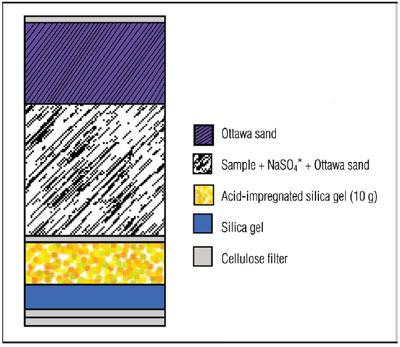
Figure 3 - Extraction cell configuration for selective extraction using silica gel.
Selective extraction of PCBs from fish tissue using alumina7
Aliquots (3 g) of fish tissue SRM (CARP-1 from the Canadian National Research Council) were ground together with sodium sulfate (15 g) using a mortar and pestle. These mixtures were transferred to ASE extraction cells containing a layer of alumina (see Figure 4). The samples were extracted automatically using the ASE system using 100% n-hexane at 100 °C with two cycles of 5 min each. The extracts were analyzed using the GC-ECD (electrochemical detection) technique. The chromatograms showed no evidence of lipids present in the extracts. The PCB recovery data are shown in Reference 7.
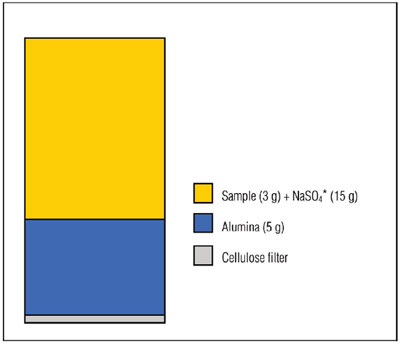
Figure 4 - Extraction cell configuration for selective extraction using alumina.
Selective extraction of PCBs from spoonbill eggs using Florisil10
Spoonbill egg samples were freeze-dried, and 2-g aliquots were spiked with 20 mL of a PCB standard solution containing 1.4 and 19 ng for low and high spike levels, respectively. Aliquots (2 g) of the spiked sample were ground with Florisil (1:2). These mixtures were added to an ASE extraction cell containing 6 g of Florisil. The samples were extracted automatically using dichloromethane (DCM):pentane (15:85, v/v) at 40 °C with two cycles of 10 min each. The extracts were analyzed using the GC-ECD technique. The results in Reference 10 compare recoveries using the ASE system with in-cell cleanup against Soxhlet extraction with postextraction cleanup with Florisil.
Conclusion
The flow-through design of the ASE system allows for in-line removal of unwanted coextractables from the final extracts, eliminating the need for a postextraction cleanup step. This ability, coupled with the speed of the extraction, makes the ASE system a powerful tool for total sample preparation.
References
- Nording, M.; Sporring, S. et al. Monitoring dioxins in food and feedstuffs using accelerated solvent extraction with a novel integrated carbon fractionation cell in combination with CAFLUX bioassay. Anal. Bioanal. Chem. 2005, 381, 1472–5.
- U.S. EPA Method 3660B, Sulfur Cleanup. U.S. Environmental Protection Agency, Cincinnati, OH, 1996.
- Dionex Corp. Determination of perchlorate in vegetation samples using accelerated solvent extraction (ASE) and ion chromatography. Application note 356; LPN 1807, Sunnyvale, CA, 2006.
- Dionex Corp. Rapid Determination of sulfonamide residues in animal tissue and infant food containing animal products using accelerated solvent extraction (ASE). Application note 353; LPN 1708, Sunnyvale, CA, 2005.
- Bjorklund, E.; Muller, A. et al. Comparison of fat retainers in accelerated solvent extraction for the selective extraction of PCBs from fat-containing samples. Anal. Chem. 2001, 73, 4050–3.
- Sporring, S.; Bjorklund, E. Selective pressurized liquid extraction of polychlorinated biphenyls from fat-containing food and feed samples influence of cell dimensions, solvent type, temperature and flush volume. J. Chromatogr. A 2004, 1040, 155–61.
- Ezzell, J.; Richter, B. et al. Selective extraction of polychlorinated biphenyls from fish tissue using accelerated solvent extraction. Amer. Envir. Lab. 1996, 12, 12–13.
- Dionex Corp. Selective extraction of PCBs from fish tissue using accelerated solvent extraction (ASE). Application note 322; LPN 0764, Sunnyvale, CA, 1996.
- Dionex Corp. Determination of PCBs in large-volume fish tissue
samples using accelerated solvent extraction (ASE). Application note 342; LPN 1204, Sunnyvale, CA, 2000. - Gomez-Ariza, J.L.; Bujalance, M. et al. Determination of polychlorinated biphenyls in biota samples using simultaneous pressurized liquid extraction and purification. J. Chromatogr. A 2002, 946, 209–19.
- U.S. EPA Method 3620C, Florosil Cleanup. U.S. Environmental Protection Agency, Cincinnati, OH, 2000.
The authors are with Dionex Corp., 1182 W. 2200 S., Ste. A, Salt Lake City, UT 84119, U.S.A.; tel.: 801-972-9292; e-mail: [email protected].