The purification and enrichment of specific cell populations from complex starting samples is a critical component of multiple life science workflows. While the key objective of most protocols is to obtain a highly pure and viable population of cells for downstream applications and analysis, most do not meet this goal.
Issues with conventional cell separation methods, such as traditional magnetic beads and fluorescent activated cell sorting, include low recovery and poor cell viability, high costs and lengthy protocol times. Magnetic beads can remain associated with the isolated cells, causing decreased cell viability and changes to cellular function, and may interfere with downstream post-processing assays. In the quest to deliver new cellbased therapeutics, it is necessary to be able to efficiently scale up cell separation workflows to process higher volumes of samples that meet emerging regulatory requirements.
Addressing the limitations of traditional cell separation techniques
QuickGel hydrogel (Quad Technologies, Woburn, Mass.) allows complete cell release from magnetic carrier particles at the end of the separation process. The addition of a biologically friendly buffer facilitates the instantaneous release of target cells from magnetic carriers. This leaves captured cells behind in their native state after removal of magnetic particles. Incubation is not required, as the gel simply dissolves in less than a minute. Cell viability is significantly increased in comparison to conventional cell separation products, and the cell capture particles that are often retained from isolated cell populations are eliminated. Importantly, the dissolution process does not affect cell phenotype or viability, which makes the isolated cells more suitable for downstream analysis and applications.
Featuring QuickGel technology, the MagCloudz streptavidin cell separation kit provides the high-efficiency capture and label-free release of high-purity target call populations from complex biological samples. The magnetic separation and dissociation process leaves isolated target cells completely label free for scalable downstream culture, analysis and functional studies (see Figure 1).
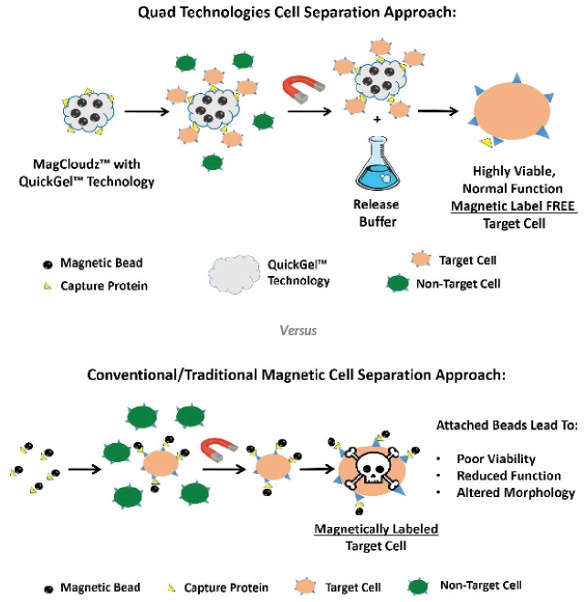 Figure 1 – Comparison of conventional magnetic cell separation methods and the MagCloudz approach.
Figure 1 – Comparison of conventional magnetic cell separation methods and the MagCloudz approach.Achieving complete cell release
MagCloudz averages ≥60% recovery of the initial target cell population and takes advantage of the high affinity of streptavidin–biotin interactions to offer biocompatibility, low nonspecific binding and high viability of magnetic, label-free, recovered target cells. The process is outlined below:
- Heterogeneous cell populations are labeled with a biotinylated antibody, directed against the cellular marker of interest (i.e., CD3-biotin for T-cell targeting).
- Biotin-labeled cell populations are incubated with the appropriate amount of MagCloudz in cell separation buffer; this allows targetcell binding to the MagCloudz surface.
- Undesired cells are removed by applying a magnetic field to the sample. The target cell–MagCloudz complexes are magnetically separated and the remaining undesired cells are removed in the supernatant.
- The target cell–MagCloudz complex is rinsed to remove any residual, nonspecifically adhered cells, further increasing target cell purity.
- The addition of proprietary release buffer gently dissolves the QuickGel, which releases the target cells and removes the magnetic carrier particles from the cells.
- A magnetic field is again applied to remove the magnetic carrier particles, which leaves the target cells behind in the supernatant.The supernatant containing target cells can then be processed as desired, achieving a highly pure and viable target cell population for further functional studies.
The kits are designed to be used for cells from mammalian sources and can process up to 100 samples containing 10 million (107) cells each, for a total of 1 billion (109) processed per kit. A maximum of 16 1.5-mL samples can be processed simultaneously using the Q-Mag magnetic stand (Quad Technologies). In some cases, processing times are shorter than conventional techniques, which require approximately two hours.
Case study: CD3+ T-cell isolation
The MagCloudz kit has been used successfully in several applications, including the isolation of CD3-positive (CD3+) T-cells from peripheral blood fractions. In one study, cell populations were stained with peridinin chlorophyll protein complex (PerCP)-conjugated anti-CD3, phycoerythrin (PE)-conjugated anti- CD45, annexin-V-FITC (apoptosis marker) and propidium iodide (viability marker) following the MagCloudz streptavidin cell separation protocol. Figure 2 shows typical data when using a biotinylated CD-3 antibody from eBioscience (San Diego, Calif.).
 Figure 2 – Representative flow cytometry dot plots for T-cell enrichment and purification from peripheral blood. a) Forward and side-scatter profile of the initial PBMC population; b) 56% CD3+/CD45+ T-cells (target cell population) were present in the initial sample; c) 92% T-cell uptake as indicated by the low percentage of T-cells remaining in the unbound cell fraction following MagCloudz streptavidin binding; d) forward and sidescatter profile of the recovered CD3+ T-cell population; e) high-purity T-cells were recovered from the MagCloudz streptavidin kit, enriched from 56% in the initial population to 98% in the final isolated/released population; and f) high viability (99%) of the recovered T-cells, indicated by the absence of annexin-V and propidium iodide staining in the bottom left quadrant of the plot. Eighty percent of the target T-cell population was recovered as magnetic label-free T-cells from the MagCloudz streptavidin kit in this assay.
Figure 2 – Representative flow cytometry dot plots for T-cell enrichment and purification from peripheral blood. a) Forward and side-scatter profile of the initial PBMC population; b) 56% CD3+/CD45+ T-cells (target cell population) were present in the initial sample; c) 92% T-cell uptake as indicated by the low percentage of T-cells remaining in the unbound cell fraction following MagCloudz streptavidin binding; d) forward and sidescatter profile of the recovered CD3+ T-cell population; e) high-purity T-cells were recovered from the MagCloudz streptavidin kit, enriched from 56% in the initial population to 98% in the final isolated/released population; and f) high viability (99%) of the recovered T-cells, indicated by the absence of annexin-V and propidium iodide staining in the bottom left quadrant of the plot. Eighty percent of the target T-cell population was recovered as magnetic label-free T-cells from the MagCloudz streptavidin kit in this assay.Conclusion
A cell separation solution that delivers highly pure and viable target cells that are magnetic label-free will significantly benefit critical research applications, drug development and cancer treatments such as stem-cell transplants and immune therapy. Researchers can easily scale up their workflows to increase throughput without compromising yield, efficiency, viability or purity.
Andrea Armstead, Ph.D., is technical marketing specialist, Quad Technologies, 3F Gill St., Woburn, Mass. 01801, U.S.A.; tel.: 339-927-9663; e-mail:[email protected]; www.quadtechnologies.com