The current standard of care for infectious disease management typically involves a nucleic acid amplification test (NAAT) to produce a definitive diagnosis. Due to the complexity of molecular technologies and instrumentation, NAATs must be performed in centralized laboratories by trained medical laboratory technologists. However, the long wait between the time the specimen is sent to the laboratory and receipt of test results by the treating physician often means that patients must be treated empirically based on clinical presentation before test results become available. In cases of asymptomatic infection, patients often go untreated.
Molecular diagnostic platform
To address this clinical challenge, the Xagenic X1 molecular diagnostic system (Xagenic Inc., Toronto, ON) can be run in a physician’s office by an untrained user during a typical office visit. It offers faster diagnosis, more targeted treatment and, ultimately, improved patient outcomes.
At the center of the system is the AuRA (amplified redox assay) molecular detection technology, which has the sensitivity and specificity of nucleic acid amplification methods but does not employ enzymes. The avoidance of enzymatic chemistry provides two advantages: 1) There is no need to refrigerate the consumables and 2) the cost of testing is reduced. The AuRA technology identifies nucleic acids through a method that involves electrochemical amplification of signal generated upon specific detection of targets of interest in a patient specimen. A self-contained, easy-to-use, disposable cartridge is configured to detect up to 60 organisms simultaneously from a single clinical specimen.
The first commercial target on this rapid testing platform is a combination test for chlamydia and gonorrhea. Both of these common sexually transmitted bacterial infections are easily cured when detected and treated. Because they are predominantly asymptomatic in patients, the best way to detect the majority of chlamydia and gonorrhea cases is through annual screening of high-risk patient populations. Left untreated, these infections can result in serious consequences such as pelvic inflammatory disease, ectopic pregnancy and infertility in women. In addition, untreated asymptomatic patients may unknowingly transmit chlamydia and gonorrhea. The U.S. Preventative Services Task Force, Centers for Disease Control and Prevention (CDC) and many medical associations thus recommend annual screening of sexually active women aged 25 and under, as well as others who are at increased risk of exposure to the causative bacteria.1
Despite annual screening recommendations and a simple course of treatment that can prevent serious complications for women, screening for chlamydia and gonorrhea (CT/NG) is suboptimal; it is believed that nearly half the population for which screening guidelines exist is actually tested.2 In some cases, physicians deem the risk to be low enough not to warrant a routine send-out diagnostic test that requires subsequent follow-up with the patient. A point-of-care (POC) testing platform that can produce a test result in 20 minutes enables proper diagnosis, counseling and treatment in the time of a single clinical visit, which should help patients and physicians adhere to best clinical practices.
 Figure 1 – Xagenic X1 system.
Figure 1 – Xagenic X1 system.The X1 system, shown in Figure 1, was designed for use by the clinician and comprises a simple, four-step process: place the specimen into the tube, insert the tube into a cartridge, enter the patient ID and, after 20 minutes, read the result (Figure 2). Due to the modular design of the testing cartridge, the X1 platform is capable of performing a multitude of diagnostic tests by detecting nucleic acids or proteins from a variety of clinical specimens.
Because the greatest clinical need for rapid testing is in infectious disease, that is where menu development will focus. Future products for the platform will include diagnostic tests for influenza A/B, Strep A, HPV, urinary tract infections, HSV 1/2, Trichomonas and HCV.
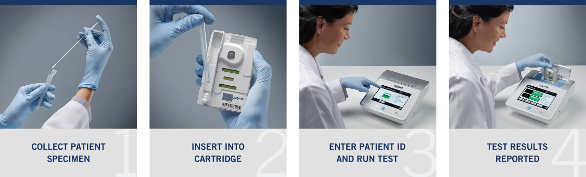 Figure 2 – Xagenic X1 performance workflow.
Figure 2 – Xagenic X1 performance workflow.Conclusion
Rapid molecular testing at the point of care will allow providers to immediately identify and implement the best course of treatment for patients, avoid incorrect use of antibiotics and prevent unnecessary, costly complications due to inadequate treatment. The Xagenic X1 rapid result molecular diagnostic platform fosters efficiencies in healthcare and timely diagnosis and treatment.
References
- www.uspreventiveservicestaskforce.org/Page/Name/uspstf-recommendations-for-sti-screening
- http://ncc.prevent.org/body/HEDIS_2012.pdf
Ihor Boszko is vice president, business development, and Shana Kelley, Ph.D., is founder and chief technology officer, Xagenic Inc., 55 York St., Ste. 1000, Toronto, ON M5J 1R7, Canada; tel.: 416-363-1999, ext. 500; fax: 416-363-1977; e-mail: [email protected]; www.xagenic.com