A portable bioanalysis system provides high resolution and high detection sensitivity, and is a cost-effective solution for the field detection of pathogens. Developed by BiOptic, Inc. (New Taipei City, Taiwan), the system utilizes direct mini-PCR technology combined with a miniaturized capillary gel electrophoresis instrument in a carry-on case to target food pathogens and disease-causing viruses within about an hour, while the operator of the unit is still at the outbreak site. The system runs in real time using batteries and can interface with a computer or smartphone that analyzes the data and displays results. Applications include clinical diagnosis of viral infectious diseases in humans, plants, and animals; border security; forensics; and environmental monitoring.
System components
The bioanalysis system comprises a mini-PCR with a programmed EEPROM with fixed parameters for specific direct PCR applications, and a compact capillary gel electrophoresis (CGE) instrument (Qsep1) for separation and fluorescence detection of the PCR products. Qsep1 integrates microcapillary electrophoretic technology into a plug-and-play, disposable, pen-shaped capillary gel cartridge with automated liquid handling, real-time fluorescence detection, and analysis. Optimized for high resolution at ambient temperatures, it provides improved peak resolution, good linear dynamic range, and reproducible migration times with a reduced instrument and sample analysis cost.
The system’s Pelican carry-on case includes the eight-sample-capacity mini-PCR device, PCR reagents, Qsep1 CGE analyzer, miniature DC air pump, and gel cartridge for the field detection of pathogens (Figures 1–3).
 Figure 1 – Field-portable bioanalysis system.
Figure 1 – Field-portable bioanalysis system. Figure 2 – Mini-PCR with eight-well sample capacity.
Figure 2 – Mini-PCR with eight-well sample capacity.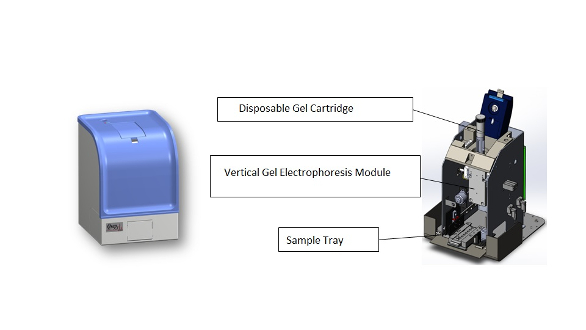 Figure 3 – Portable capillary gel electrophoresis (Qsep1).
Figure 3 – Portable capillary gel electrophoresis (Qsep1).Results
ALDH2 gene mutation
Mitochondrial aldehyde dehydrogenase is an enzyme encoded by the ALDH2 gene located on chromosome 12 in humans. Aldehyde dehydrogenase is the second enzyme of the major oxidative pathway of alcohol metabolism. Two major liver isoforms of aldehyde dehydrogenase, cytosolic and mitochondrial, can be distinguished by their electrophoretic mobilities. Direct PCR saves time and money by allowing the operator to omit DNA extraction and purification and go directly to PCR amplification. To evaluate system suitability, BiOptic formulated DirectGO PCR Master Mix for the ALDH2 gene mutation test to determine an individual’s alcohol tolerance directly from saliva.
Figure 4 is the result for the PCR Primer N as control, showing that all individuals (A–F) have at least one normal gene. Figure 5 is the test result for Primer M, which shows that individual B has a distinct mutation with the lowest tolerance, and individuals A, C, and F have the highest tolerance to alcohol consumption.
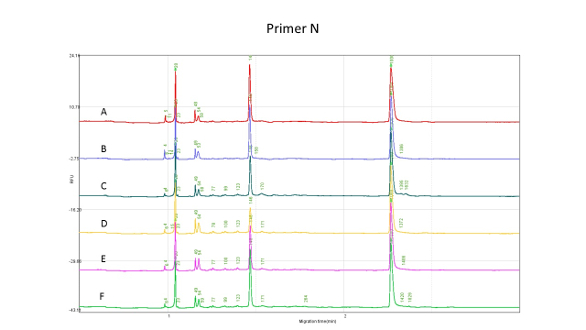 Figure 4 – PCR Primer N as
Figure 4 – PCR Primer N as control
for alcohol tolerance test (ALDH2 gene mutation) showing that all individuals (A–F) have at least one normal gene.
Figure 5 – PCR Primer M test results showing that individual B has distinct mutation with the lowest tolerance and individuals A, C, and F have the highest tolerance.
For the ALDH2 gene mutation test, the DNA from saliva was amplified in 50 minutes by the DirectGO direct amplification kit using the mini-PCR. The PCR product was then separated and detected with the ready-to-use gel cartridge, which can be set up in 1 minute, and produces results in 3–5 minutes.
Additional reading
- http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0082704
- BiOptic DirectGO PreMix; catalog no. C108200.
The authors are with BiOptic, Inc., New Taipei City, Taiwan (ROC), and BiOptic, Inc., 1409½ Foothill Blvd., La Canada Flintridge, Calif. 91011, U.S.A.; tel.: 818- 679-4413; e-mail: [email protected]; www.bioptic.com.tw