Growers routinely test for pesticides in food. The United States Department of Agriculture (USDA) through the Pesticide Data Program (PDP) and other agencies do the same, in order to ensure that pesticide levels are below tolerances set by the U.S. Environmental Protection Agency (EPA).1
Fresh and processed fruits and vegetables are the primary food commodities tested by the PDP.1 More than 10,000 samples were collected randomly from the U.S. food distribution system in 2013 and subsequently tested for over 300 pesticides, 57 of which were detected and quantified in at least 5% of one or more commodity products.1 In typical methods for pesticide quantification, such as QuEChERS,2 at least four calibration standards are required per analyte. Thus, over 200 calibration standards must be analyzed before concentrations of the 57 detected pesticides can be determined. These procedures result in long and costly analyses and significant potential for error associated with incorrect preparation of calibration standards.
Gas chromatography is commonly used to quantify pesticides. In GC, a sample is injected into the system and rapidly vaporized; the chemical components are separated from each other as they flow through specially designed tubes or capillary columns. The separated chemicals flow into a detector that produces a signal specific to each chemical compound in the mixture. This detector signal appears as a peak when shown as a function of time. Detection systems used for the quantification of pesticides include the flame ionization detector (FID), electron capture detector (ECD), flame photometric detector (FPD), nitrogen phosphorus detector (NPD) and mass spectrometer detector (MSD). All detectors produce responses (i.e., signals per mole of analyte) that are different for each molecule and, in general, are unknown without calibration. Calibration is time consuming and must be carefully performed before quantitative results can be obtained. However, the new detection system for implementation with GC/FID described in this article does not require calibration, resulting in the fast and accurate quantification of pesticides.
Detection system converts organic compounds to methane
 Figure 1 – Polyarc reactor for calibration-free GC/FID analyses.
Figure 1 – Polyarc reactor for calibration-free GC/FID analyses.In this study, a Polyarc reactor (Activated Research Company, Eden Prairie, Minn.) (Figure 1) was used to convert organic molecules to methane at the exit of the GC column (Agilent 7890A GC with DB-5, 30 m, 0.32 mm, 0.25 μm film thickness column, Agilent Technologies, Santa Clara, Calif.) equipped with an FID and split/splitless inlet. The reactor temperature setpoint and flow rates were 293 °C, 35 std. cm3 min–1 H2 and 2.5 std. cm3 min–1 air. FID temperature and flow rates were 300 °C, 1.5 std. cm3 min–1 H2 and 350 std. cm3 min–1 air.
To quantify the amount of a component in a mixture, such as a pesticide in food, the relationship between detector signal and concentration must be determined. Most chromatographers use the terminology response factor (RF) when referring to the response of an analyte compared to an internal standard. Response factors are determined from the analysis of calibration standards and are used to identify the unknown concentrations of particular molecules in samples of interest. Response factors are calculated for each analyte from the integrated detector signal using the formula below:

where 1 and 2 are the analyte and internal standard, and area is the integrated detector signal as a function of time for that component (i.e., areas of the peaks). mol C are the injected moles of carbon of the component.
The response factors for all compounds are equivalent
Figure 2 shows the pesticides tested with GC/FID using the Polyarc reactor. All have been restricted or banned by the EPA for use on food crops. Atoms other than C—including N, O, P, S and Cl—are present in these molecules, and typically cause the FID response factors to vary significantly.3 Even with the theory that exists for estimating response factors,4 most researchers choose to experimentally determine FID response factors using calibration standards, because variations in instrumental setup can lead to deviations from theory.
 Figure 2 – Molecular structures of the pesticides tested using GC/FID with the Polyarc reactor.
Figure 2 – Molecular structures of the pesticides tested using GC/FID with the Polyarc reactor.The response factors for the pesticides tested using the Polyarc reactor are shown in Figure 3 with error bars that represent the uncertainty, including gravimetric uncertainty and GC peak area uncertainty. Methanol was used as the solvent and internal standard for these measurements. The response factors for all of the compounds were 1.0 ± 0.04. The mean deviation between the response factors and unity was 1.8%. The reactor was tested with different pesticide concentrations to determine the linearity of response and limits of detection. The measured concentrations (determined with RF = 1) and actual concentrations of the pesticides (Figure 4) demonstrate linearity and accuracy of the detector response over three orders of magnitude. Based on the smallest possible peak area distinguishable from noise and the largest solvent peak area measured in this study, the Polyarc reactor gives a linear detector response over eight orders of magnitude in concentration.
 Figure 3 – GC/FID response factors for six pesticides analyzed with the Polyarc reactor.
Figure 3 – GC/FID response factors for six pesticides analyzed with the Polyarc reactor. Figure 4 – Measured concentrations of analytes using the Polyarc reactor (with RF = 1) compared to actual concentrations of prepared samples (gravimetric) for nine samples of the pesticides shown in Figure 2.
Figure 4 – Measured concentrations of analytes using the Polyarc reactor (with RF = 1) compared to actual concentrations of prepared samples (gravimetric) for nine samples of the pesticides shown in Figure 2.The response provided by the reactor is proportional to the number of carbon atoms in each molecule, despite the large differences in their chemical formulas and functionalities. The N, O, S, P and Cl atoms present in the pesticides have no effect on the response of the FID per carbon atom, because the molecules are completely converted to CH4 in the reactor. This is a marked difference from the response variability expected for these molecules in a traditional FID.3 Thus, calibrations to determine response factors are not necessary with the Polyarc reactor, since the response factors for all organic compounds reflect those of methane and are therefore equivalent.
Detecting malfunctions with GCs
A benefit of a calibration-free system is its ability to detect problems with the setup of GCs. The accumulation of small amounts of an analyte within the inlet, column or other location of a GC is known as “hold-up,” and this often increases with system use as the flow path becomes dirty or less inert. It can result in inaccurate results, especially with dilute analyte concentrations, where the problem is accentuated. For example, if the response factor of an analyte is determined with a dirty system in which analyte hold-up occurs, the measured response factor will be artificially low and could depend nonlinearly on the concentration of the analyte and other compounds in the mixture. This can lead to inaccuracies when the response of the analyte is not completely known. Further, if the system is cleaned and hold-up is eliminated, artificially high concentrations will be reported.
Figure 5 shows the results of injecting small masses of captafol into a liner in which holdup takes place using a GC/FID equipped with a Polyarc reactor. At low analyte masses, the response factor is less than 1, presumably because a large fraction of the analyte is adsorbed in the liner. Since a properly functioning instrument would give a response factor of one, it is clear that there is a problem with the injection system. Subsequent action can be taken to resolve the problem before unknown samples are analyzed. With traditional calibration and analysis techniques, this problem would go unnoticed and could result in inaccurate quantification of compounds.
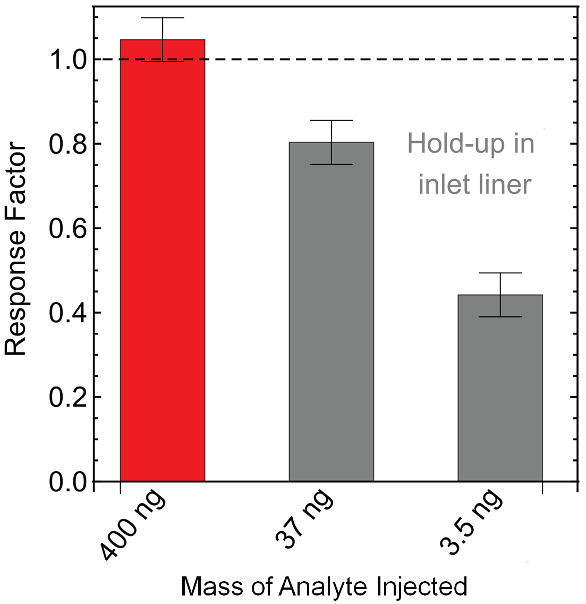 Figure 5 – Artificially low response factors are seen for small injection masses of captafol due to hold-up in the inlet liner.
Figure 5 – Artificially low response factors are seen for small injection masses of captafol due to hold-up in the inlet liner.Conclusion
Traditionally the process of quantification of pesticides in food requires analysis of many calibration standards before the concentrations of unknowns can be determined. By coupling a GC/FID system with a catalytic microreactor in which all organic compounds are converted to methane, the response factors are equivalent and calibration is no longer required. This leads to faster analysis times, lower costs and reduction in the number of errors. The reactor system is able to detect and remedy hold-up or other GC issues that cause inaccuracies.
References
- Pesticide Data Program—Annual Summary, Calendar Year 2013. United States Department of Agriculture, Agricultural Marketing Service 2014; http://www.ams.usda.gov/sites/default/files/media/2013%20PDP%20Anuual%20Summary.pdf
- Lehotay, S.J. Quick, easy, cheap, effective, rugged, and safe approach for determining pesticide residues. In Pesticide Protocols, Springer: New York, N.Y., 2006; pp 239–61.
- Dietz, W.A. Response factors for gas chromatographic analyses. J. Chromatogr. Sci. 1967, 5(2), 68–71.
- Scanlon, J.T. and Willis, D.E. Calculation of flame ionization detector relative response factors using the effective carbon number concept J. Chromatogr. Sci. 1985, 23(8), 333–40.
- Maduskar, S.; Teixeira, A.R. et al. Quantitative carbon detector (QCD) for calibration- free, high-resolution characterization of complex mixtures. Lab on a Chip 2015, 15(2), 440–7.
Charlie Spanjers is a post-doctoral associate, and Paul Dauenhauer is a professor of Chemical Engineering and Materials Science, University of Minnesota, Amundson Hall, 421 Washington Ave. SE, Minneapolis, Minn. 55455, U.S.A.; e-mail: [email protected].
Professor Paul Dauenhauer and a team of researchers at the University of Minnesota wanted a faster and more accurate method to determine the concentrations of organic compounds, so they used a newly developed technology, the Polyarc reactor. The Polyarc reactor is a catalytic microreactor that converts organic compounds to methane at the exit of the GC and before detection in the FID. To optimize separation efficiency, the device has specially designed microchannels with catalysts to ensure full conversion of organic molecules to methane and a unique application of 3-D printing with stainless steel is used to create the reactor cartridge. Because FIDs are only sensitive to compounds containing carbon, the Polyarc reactor makes the response per carbon atom equivalent for all compounds. A detailed investigation of the device was recently published by the Catalysis Center for Energy Innovation (CCEI),5 and new applications for the reactor are being explored.