Water purity is a fundamentally important factor in many laboratories, yet it is frequently overlooked. Researchers use water in most lab processes, and poor water quality can quickly lead to mounting problems, resulting in the generation of inaccurate results. This is especially pronounced in studies that make use of highly sensitive analytical processes such as chromatography and spectroscopy, in which even low levels of contamination can skew the output of an experiment. Ensuring water is of the right quality for the task at hand, as well as the ability to detect contaminants when present, are both exceedingly important considerations when carrying out most types of scientific research in the laboratory.
Common types of water contamination
 Figure 1 – Common contaminants found in water prior to purification.
Figure 1 – Common contaminants found in water prior to purification.There are several general types of possible contamination that can affect lab water purity (also see Figure 1).
Suspended particles
The most conspicuous form of contamination, suspended particles or particulate matter, includes anything from silt and vegetation to colloids and various pollutants. Suspended particles can result in water turbidity, which is quantified using a nephelometer, whereby the scattering of a light beam as it passes through the water is quantified by measuring the amount of light reaching a detector (reported as Nephelometric Turbidity Units, NTU). Suspended particles are typically extracted by filtration, ensuring that anything above a designated size is removed from the water long before laboratory use.
Dissolved compounds
Dissolved compounds are present in both organic and inorganic forms. Dissolved inorganic compounds make up the bulk of the impurities found in water and are principally mineral in nature (sodium, iron, calcium, nitrate, sulfate, etc). To assess the extent of dissolved inorganic compounds, a water sample is evaporated at 180 °C and the inorganic salts left behind are used to infer the level of inorganic compounds present in the water source. Individual inorganic constituents can subsequently be determined and quantified by ion chromatography (IC), inductively coupled plasma-mass spectrometry (ICP-MS), or other spectrophotometric methods, if required.
Unlike inorganic compounds, dissolved organic compounds are biological in nature and, if left unchecked, can very efficiently support the growth of microorganisms. Total organic content (TOC), sometimes referred to as total organic carbon, is the most common way to measure and report the level of dissolved organic compounds in water. The TOC value is calculated by quantifying the oxidation products generated from a water sample following a process that involves acidification followed by combustion at high temperatures in an oxygen-rich atmosphere. It can also be measured inline by correlating the resistivity of a water sample with a known reference standard.
Microorganisms
Microbial contamination can be a complex issue; not only do the bacteria themselves represent a clear problem, but they also release compounds such as endotoxins and nucleases, which are capable of disrupting numerous experiments. Bacterial levels are typically reported as colony forming units per milliliter (CFU/mL), assessed following culture on selective media. However, rapid procedures involving spectrophotometric detection of bacterial contamination are now being used more frequently for inline detection.
The endotoxins released from the cell wall of gram-negative bacteria (reported as endotoxin units per milliliter, EU/mL) can be assessed using standard tests based on the Limulus Amebocyte Lysate (LAL) assay. The LAL method of endotoxin determination relies on the interaction of a proenzyme (Factor C) extracted from horseshoe crab blood cells (amebocytes), with the endotoxins present in the sample. The lipopolysaccharide endotoxin activates the proenzyme to produce a yellow color in the presence of a synthetic substrate (p-nitroaniline). The relative amount present can be determined by using a spectrophotometer to measure the absorbance at 405 nm.
Ionic content
Ionic content is a direct contributor to pH. However, stray ions can also disrupt sample analysis under certain conditions (during IC and MS, for example). The pH itself can be a tricky parameter to measure, especially in very pure water, which by definition has an exceptionally low ionic content. Instead, conductivity is used to measure the ability of a water sample to conduct electrical current (reported as microSiemens per centimeter, μS/cm). Typically used to analyze relatively low-purity water, conductivity provides a nonspecific indication of the water’s ionic content. For an ionic content assessment at much finer resolution—as is required for a very pure water sample—resistivity is used instead, reported as mega-ohms per centimeter (MΩ-cm). In order to determine conductivity and/or resistivity, water is continually passed through an electric meter or cell to accurately determine these values. Temperature will affect these parameters; thus, ensuring a thermostable environment during the assessment is crucial.
Impact of water contamination on research
Failure to accurately determine the quality of water can have potentially disastrous consequences for numerous experimental procedures (Table 1). Suspended particles and particulate matter can accumulate, blocking equipment such as filters or columns. Organic and inorganic content can easily be introduced into what would be an otherwise pure system by rinsing equipment with insufficiently pure water between experiments. Dissolved inorganic material may result in ionic instability, potentially disrupting normal protein–protein or protein–lipid interactions, which in turn can influence the overall rates of chemical reactions. Organic compounds can interfere with blotting techniques by upsetting the hybridization process on account of nonspecific binding, or even compete with the analyte in the mobile phase during analytical processes such as high-performance liquid chromatography (HPLC), lowering the overall sensitivity of the analysis.
Table 1 – Consequences of several water contaminants on various scientific procedures
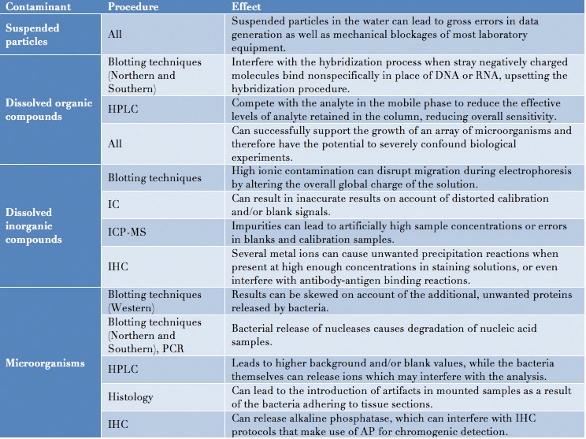 Key: HPLC = high performance liquid chromatography, IC = ion chromatography, ICP-MS = inductively coupled plasma mass spectrometry, IHC = immunohistochemistry, PCR = polymerase chain reaction.
Key: HPLC = high performance liquid chromatography, IC = ion chromatography, ICP-MS = inductively coupled plasma mass spectrometry, IHC = immunohistochemistry, PCR = polymerase chain reaction.
Bacteria present in lab water will have very obvious consequences for anyone working in microbiology or cell culture, but a more subtle effect is caused by the nucleases they release: by breaking down nucleic acids in a sample, these nucleases will quickly bring molecular biology experiments using DNA and RNA to a standstill. Finally, bacterial accumulation can also lead to the development of biofilms, representing an ongoing source of contamination that can persist for many years.
It is also worth noting that different lab applications require varying levels of water purity (Figure 2); selecting the correct water type for each is important, both to help minimize costs (by only using ultrapure water when necessary) and to ensure that any contamination present will not impact on the application at hand.
 Figure 2 – Different lab applications require varying levels of water purity.
Figure 2 – Different lab applications require varying levels of water purity.Avoiding water quality pitfalls using in line monitoring
As discussed here, maintaining the correct level of water quality should be a priority for all lab work. While determining the initial level of purity is essential, ensuring that that this level is being maintained is just as important. The optimal approach is through continuous monitoring of the water output via inline sampling equipment built directly into the purification system. For example, the addition of sensors for monitoring resistivity and TOC can save time and money by eliminating the need for laborious offline testing. With advanced purification technologies now available that have inline monitoring systems for many of these contaminants built-in, such as those produced by ELGA LabWater (Woodridge, IL), it is possible to have access to ultrapure water “on demand” without having to worry about the negative impact of unexpected contamination spikes.
Conclusion
It is imperative that all the reagents used in the lab are of the highest possible standard if the data produced are to be robust, reliable, and accurate. Overlooking something as seemingly innocuous as water purity can quickly disrupt experiments, wasting time and money, especially since water plays a role in so many lab processes and experimental procedures. In the first instance, be sure to select the right water purity for the application in question. Next, be aware of the potential types of contamination and how to test for them. Finally, save yourself the time, hassle, and worry of continually needing to test the purity of your water supply by using purification systems that have built-in water monitors.
Jim Camilleri is the General Manager for ELGA LabWater in North America. 5 Earl Court, Suite 100, Woodridge, IL 60517 USA; tel.: (877) 315-3542; fax: (630) 910-4798; e-mail: [email protected]; elgalabwater.com