Synthetic cannabinoids are attracting increasing attention from the drug testing industry because of a sudden surge in the abuse of these compounds. The situation is so severe that an emergency ban of five synthetic cannabinoids was enforced by the U.S. Drug Enforcement Agency (DEA) in the spring of 2011.1 As a result, toxicology labs are scrambling to develop analytical methods to detect these compounds. This article describes a study designed to develop a method combining streamlined extraction from a urine matrix followed by fast, sensitive LC/MS/MS analysis.2–4
Figure 1 - Synthetic cannabinoids analysis.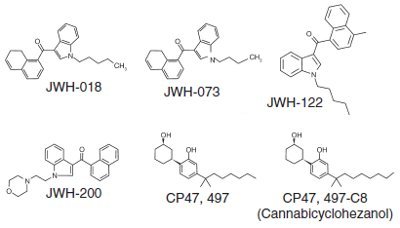
Table 1 - Solid-phase extraction of synthetic cannabinoids from urine using Strata-X-Drug N

Table 2 - LC/MS/MS analysis of synthetic cannabinoids

Experimental
Forensic toxicology labs are frequently under extreme time limitations to develop new methods for abused substances when they are identified as critical problems. This time sensitivity was taken into account when developing a solid-phase extraction (SPE) method to swiftly analyze the five synthetic cannabinoids that have been banned by the DEA plus a sixth—JWH-122—which has also been banned by several states (Figure 1).
Synthetic cannabinoids are excreted in urine as glucuronide conjugates that require a pretreatment step prior to SPE cleanup. The pretreatment involves a hydrolysis step to remove the glucuronide from the compounds, enhancing extraction and detection by LC/MS/MS.
Sample pretreatment
To 2 mL of urine, 1000 μL of β-glucuronidase solution (containing 5000 F units/mL Patella vulgata in 100 mM acetate buffer, pH 5.0) is added. The mixture is then allowed to hydrolyze for 3 hr at 60 °C. The samples are then allowed to cool for 5 min, after which 1000 μL of 100 mM phosphate buffer (pH 6.0) is added, verifying that the pH is between 5.5 and 6.5. Samples are then centrifuged for 5 min at 5000 rpm and the pellet is discarded.
The Strata™-X-Drug N reversed-phase polymeric SPE sorbent was utilized (see Table 1). Strata-X-Drug N differs from other SPE sorbents in that it eliminates the condition/equilibration step without sacrificing recovery. The sorbent is also quality control (QC) tested with drugs-of-abuse probes from actual urine samples to ensure that the product performs as expected in reallife situations.
Table 3 - Absolute recoveries of synthetic cannabinoids from urine


Figure 2 - LC/MS/MS chromatogram of extracted synthetic cannabinoids.
LC/MS/MS analysis
After the synthetic cannabinoids were successfully cleaned and concentrated, the eluent was analyzed by LC/MS/MS using a Kinetex® 2.6 μm PFP core-shell HPLC/UHPLC (ultrahigh-performance liquid chromatography) column (Phenomenex, Torrance, CA) coupled to an API 4000 MS/MS (AB SCIEX, Ltd., Foster City, CA). Deuterated standards were also analyzed alongside the extracted compounds to verify proper quantitation and detection (see Table 2).
Results
After analyzing several different wash strengths during the SPE procedure, it was determined that the Strata-X-Drug N sorbent was able to retain the synthetic cannabinoids so tightly that it could undergo a 50% methanol wash without loss of analyte. This produced a clean extract while still providing high recoveries (Table 3). Most reversed-phase SPE methods specify a 5–10% methanol wash to prevent analyte loss. The elimination of the condition/equilibration step also provided time and solvent savings without affecting analyte recovery.
Table 4 - Polarity switching and multiple reaction monitoring (MRM) transitions

The method used to analyze the synthetic cannabinoids was slightly more challenging because it required scheduled polarity switching between ESI (+) and ESI (–). Because polarity switching was required, it was essential that there be complete separation between analytes. The Kinetex 2.6 μm PFP core-shell HPLC/UHPLC column provided very good separation of analytes in under 4 min (Figure 2), which allowed polarity switching and time savings (Table 4).
Once the LC/MS/MS method was developed, linearity and reproducibility were analyzed by performing six-point calibration curves at varying concentration levels (0.1, 0.5, 2.0, 5.0, 50, and 100 ng/mL). All six synthetic cannabinoids provided correlation coefficient values that equaled 0.999 and above (Table 5), proving that the LC/MS/MS method was not only sensitive but also reproducible.
Table 5 - Linearity of LC/MS/MS method

Conclusion
As the use of synthetic drugs spreads, laboratories must quickly adapt by developing rapid, sensitive, and reproducible extraction and analysis methods. The work described here demonstrates high-recovery, cost-saving extraction combined with sensitive, reproducible LC/MS/MS for the analysis of six synthetic cannabinoids.
References
1. Health Experts Warn Against “Fake Pot,” NBC Chicago, June 1, 2010; http://www.nbcchicago.com/news/local/k2-fakepot- 94962744.html.
2. Chemicals Used in “Spice” and “K2” Type Products Now Under Federal Control and Regulation, United States Drug Enforcement Administration, March 1, 1011; http://www.justice.gov/dea/pubs/ pressrel/pr030111.html.
3. Synthetic Cannabinoids and “Spice,” European Monitoring Centre for Drugs and Drug Addiction; http://www.emcdda.europa.eu/publications/ drug-profiles/synthetic-cannabinoids.
4. National Conference of State Legislators; http://www.ncsl. org/?TabId=21398.
Erica Pike, M.A., is Sample Preparation Brand Manager, Michael Rummel, B.Sc., is Sample Preparation Product Manager, Matthew Trass, B.Sc., is Application Specialist, Jeff Layne, Ph.D., is Senior Research Scientist, and Sky Countryman, B.Sc., is Manager of PhenoLogix and Applied Technologies, Phenomenex, Inc., 411 Madrid Ave., Torrance, CA 90501, U.S.A.; tel.: 310-212-0555; e-mail: [email protected].