The basis for observations has not really changed in
all the time that lenses have been used to magnify
objects. Illumination is required to power the imaging
system, preferably a light source that can be
moved and focused, and powerful lenses are used to
magnify and separate the specimen.
Ernst Abbe’s brilliant theory of image formation laid
down the scientific basis for the design and manufacture
of microscopes over 130 years ago. The introduction
of apochromatic lenses and oil immersion
enabled light microscopes to reach their theoretical
limit of resolution of approximately 200 nm. Then,
in 1893, Köhler’s optimized illumination system
allowed microscopic specimens to be imaged with
absolute homogeneity and maximum contrast. A
comparison of any modern microscope to the original
Carl Zeiss model (Herts., U.K.) makes the
ancestry obvious.
If the fundamentals of microscope design have not
changed in 110 years, have microscope makers and
users reached a scientific cul-de-sac? A simple
glance through the scientific literature quickly dispels
this view. Modern microscope optics, epitomizing
brightness and resolution, have been augmented
by the introduction of laser illumination
systems and sophisticated digital image processing software. Meanwhile, the development of fluorescent
labels capable of targeting specific areas of the
cell without altering its processes means that high-resolution fluorescence microscopy has become a
key tool in cell studies.
Biologists can now visualize the dynamics of the
cell’s biological processes in high contrast and
minute detail, even down to the single molecule
level. However, for many routine users of fluorescence
and phase contrast microscopy, image quality
is problematic. Many research groups find that budgetary
considerations inhibit the scope of their work.
Innovative imaging technologies specially designed for
everyday use are now available to tackle these problems.
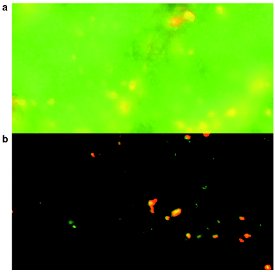
Figure 1 - Neuronal tissue of the supraoptic nucleus of the
rat visualized by two-channel fluorescence in conventional
mode (a) and in ApoTome mode (b) (Carl Zeiss).
In fluorescence microscopy, samples
incorporating a fluorophore are illuminated
with light of one wavelength,
and the higher wavelength
light that is released is captured.
The problem is that light emitted
by fluorescent material that is not
of interest can overwhelm the
image information the user is trying
to capture. This is especially true in
thicker samples, such as tissue
slices, where image information
from above and below the focal
plane affects the level of detail visualized
within the objective's depth
of field (Figure 1a).
A confocal laser-scanning microscope
plus powerful PC and specialized
imaging software, such as the
AxioVision Inside4D suite (Carl
Zeiss), can eliminate the blur by optically
slicing the sample and removing
the parts of the image that are out of
focus (Figure 1b). However, there is
another way. Called Structured
Illumination, it is incorporated into
the ApoTome mode.

Figure 2 - Fluorescein isothiocyanate (FITC)- and rhodamine-labeled cells contrasting traditional
epi-fluorescence illumination (a) with the image with the ApoTome slide in place (b).
Capable of resolving to one Airy
unit section depth, ApoTome is a
small, discrete slider that fits into the Axiovert
200 and Axioplan 2 microscopes (Carl Zeiss) to
provide an immediate improvement in image
quality. It increases visible resolution by 100%
and displays full 3-D images of thick optical sections
while maximizing contrast.
Because ApoTome eliminates the time-consuming
and costly process of making optical sections
of biological specimens, it brings this high-performance
microscopy technique to many more
users. The advance is crucial in areas such as cell
research, where the improvement to resolution
and contrast in thick specimens is vital. Optical
sections are also a major requirement for 3-D
reconstructions, and ApoTome produces 2-D and
3-D images free of stray light, with high signal-to-noise
ratios (Figure 2).
Principle of operation
The development of the ApoTome is based on the
principle of fringe projection. While this approach is
not new, the unit is reliable, artefact free, and ready
for daily work in digital imaging.

Figure 3 - Schematic representation of the ApoTome imaging principle: a) Blurred
specimen, b–d) specimen with grid pattern overlay in three positions, and e) resulting
image as optical section with increased contrast and sharpness.
The ApoTome slider eliminates blurring by projecting
a grid pattern into the beam path (Figure 3a)
that is moved automatically through three positions
under software control (Figure 3b–d). When the
images are automatically combined with a fast
mathematical algorithm, the grid pattern is eliminated
and clear, high-contrast 3-D information is
displayed (Figure 3e). The entire process takes less
than 80 msec and, in comparison with the long
postprocessing normally associated with optical
sectioning, this is optical sectioning in real time.
The imaging system can be used for a wide range of fluorescence imaging applications in which high
image quality and resolution are important. The system
also gives users almost unlimited freedom in their
choice of fluorochromes. Whether the methodology
requires 4’,6-diamidino-2-phenylindole (DAPI),
FITC, rhodamine, or a living dye such as green fluorescent
protein (GFP) or yellow fluorescent protein
(YFP), ApoTome will significantly improve definition
and contrast. It is particularly well suited to analyzing
fixed samples with a specific fluorescent stain
(e.g., immuno-labeling) in combination with high-resolution,
high-numerical aperture objectives.
Fluorescence at the cell
surface
Many of the key events in the cell occur in close
proximity to membrane surfaces or at the surface
of the cell. Naturally, any optical technique that
can visualize these events without interference
from the underlying regions within the cell or cellular
structure will increase the amount and quality
of information collected.
Total internal reflection fluorescence (TIRF)
microscopy has played a key role in helping us understand
the myriad of cellular processes occurring at the
cell surface. Often referred to as evanescent wave
microscopy, it can enable the direct observation of
membrane fusion of synaptic vesicles and the movement of single molecules during signal transduction. It
is not a new technique, but has surged in popularity
with the advent of GFP as a fluorescent marker and
technical developments in laser scanning microscopes.
With the Simple Internal Reflection Fluorescence
(SIRF) system (Carl Zeiss), individual scientists can utilize
a total reflection microscope system based exclusively
on standard HBO/XBO light sources rather than lasers.